Monday, August 28, 2006
The Vatican's Intelligent Designs?
To Clone or Not to Clone: Is that the question?
A Moderated Panel Discussion
Moderators:
Kathryn Hinsch, Founder, Women's Bioethics Project
Petra Franklin Lahaie, Board Member of both Women’s Bioethics Project and ACT Theater
Quote from Kathryn Hinsch, Founder, Women's Bioethics Project: “A key part of the Women’s Bioethics Project’s mission is to help people understand the implications of merging technologies.” said Kathryn M. Hinsch, founder of the Women’s Bioethics Project. “To some, cloning might seem like science fiction. But, in fact, last year, more than 500 bills were introduced at the state level that related to cloning or stem cell research. Since there is currently no federal law banning human cloning, we expect it will be a hot topic in 2006. Just this past year, California, Connecticut, and Massachusetts each passed legislation banning human cloning, but permitting the technique for research purposes. It is something voters need to know about now”
For more, check out the WBP home page.
Friday, August 25, 2006
New technique for developing embryonic stem cells: Does it make a moral difference?
Proponents of the new technology say that it eliminates the major objection of those opposed to embryonic stem cell research: it doesn't destroy embryos. Some disagree, however, arguing that the single cell that is removed also has a right to life. The Vatican, which has long opposed all IVF technologies, says that the new method is unacceptable.
Still others doubt that technological changes can answer the inescapable ethical/moral question of whether embryonic research should be permissible. As Art Caplan told the New York Times (see the third link below), "This isn't a technique that's going anywhere. This isn't an alternative to anything."
Read more all over the place: here, here, and here, for starters.
FDA Approves Plan B's Over-the-Counter Sale
From the Washington Post this morning:
By the end of the year, American women will be able to walk into any pharmacy and buy emergency contraceptive pills without a prescription as a result of a Food and Drug Administration decision announced yesterday.
The decision means women will not have to go to a doctor first as long as they can prove they are 18 or older to a pharmacist, who will keep the drugs behind a counter. Younger teenagers will still need a prescription, and the pills will not be sold at gas stations, convenience stores or other outlets that do not have pharmacists.
The approval marks the first time a hormonal contraceptive will be broadly available in the United States without a prescription. The pills, which will be sold as Plan B, will probably cost about $25 to $40 per dose, and men will also be able to buy them.The announcement was aimed at resolving one of the longest and highest-profile health controversies of the Bush administration, but opponents said they are considering plans to block the decision, either in court or in Congress.
To read on, click here.
Thursday, August 24, 2006
Is meat-packing better regulated than the tissue transplant industry?.
As organ and tissue transplantation has become more viable and more commonly practiced, the demand for donor tissues and organs has increased. An industry of companies that harvest and provide tissues for transplant has surfaced to meet this demand. Almost any kind of tissue may be handled by this industry, including organs, skin, and bone. So how exactly is it that companies who provided this service were not required to register with the FDA until 2004?
It seems that prior to 2004 regulation of transplant tissues paled in comparison to that of the meat-packing industry. How is it possible that tissues that we eat and pass through our bodies are better screened than those that are placed permanently in our bodies? While my knowledge of the immune system is far from up to par, it seems to make biological sense that its easier for my immune system to fight off a population of bacteria originating from a food that passes through the digestive system, as there it will at least be partly processed and degraded, unlike a bacterially infected tissue that takes permanent home with other bodily organs and tissue.
New developments have surfaced since first beginning this writing in July 2006. Just this month, the FDA ordered the human tissue recovery firm Donor Referral Services (DRS) to shut down its operations, finding “serious deficiencies in its manufacturing practices, including those governing donor screening and record keeping.” I find this astounding. It seems that at every suspect outbreak of mad-cow disease, there is a barrage of public health warnings and media hype, quickly followed by indications that the disease path as been tracked and the origination determined and quickly shut down. Amazingly, records and manufacturing processes for tissue recovery and donation seem much looser. Case in point is the instigation of the shut down of DRS: “FDA's inspection identified serious violations of the regulations, including the failure to establish and maintain procedures for manufacturing steps...FDA also found several instances where records provided by DRS to another HCT/P establishment were at variance with the official death certificates FDA had obtained from the state where the death occurred.”
The FDA's new regulations for tissue recovery and transplant services took effect in May 2005. “Among other mandates, the regulations require firms to properly screen and test donors and, when needed, they enable FDA to take swift action in the interest of public health.” Better late than never I suppose; but I can't help but suspect that both the industry and the FDA is saving face for not implementing preemptive regulation long ago.
New regulations for donor eligibility and screening can be viewed here: http://www.fda.gov/bbs/topics/news/2004/NEW01070.html
Press release on DRS shutdown: http://www.fda.gov/bbs/topics/NEWS/2006/NEW01433.html
Tuesday, August 22, 2006
Experimental treatments: a constitutional right?
From the London Times Online:
Giving unproven drugs to terminal patients won't save lives in the end.
Science Notebook by Anjana Ahuja
ABIGAIL BURROUGHS, a 21-year-old American, died from squamous-cell carcinoma of the head and neck in 2001. According to her father, Frank, she didn’t need to die. There were drugs in development that might have reined in the malignancy, but the experimental therapies were being tested on cancers in other parts of the body.
“She had the right cells in the wrong place, and she didn’t qualify for any of the clinical trials,” her father recalls. Shortly after her death, he founded the Abigail Alliance for Better Access to Experimental Drugs. This summer it scored a breathtaking victory in the courts against the Food and Drug Administration. The District of Columbia Circuit ruled, by two to one, that a patient with a terminal illness or untreatable disease had a “fundamental right” under the Constitution to experimental drugs that have passed only preliminary (Phase I) tests, and are thus a long way from approval.
The little-reported decision is already having deep repercussions in the medical world, and is likely to end up in the Supreme Court. While patients welcome the decision, regulators and clinical researchers fear that such early, wide access to experimental drugs will make it harder to obtain the clear, long-term data needed to prove efficacy. Patients may decide an unproven drug is a better gamble than ending up on a proven, but marginally effective, medication. An article in the New England Journal of Medicine this month points out that only 11 per cent of drugs — and only 6 per cent of cancer drugs — that enter clinical testing are ultimately approved; the rest are either too toxic or don’t work.
To read on, click here.
Quote of the Day: "Politicians sell terror and fear; pharmaceutical companies sell disease."
By Jonathan Rowe
POINT REYES STATION, CALIF. – It is an old saying in the advertising trade that you sell the problem, not the solution. That helps explain why the media today are awash with images of disease. Erectile dysfunction, depression, stress, attention deficit disorder, on and on - you can't escape them and the sense of looming peril that they conjure up.
Politicians sell terror and fear; pharmaceutical companies sell disease. Every state and stage of existence has become a pathology in need of pharmaceutical "intervention," and life itself is a petri dish of biochemical deficiency and need. Shyness is now "social anxiety disorder." A twitchy tendency has become "restless leg syndrome." Three decades ago the head of Merck dreamed aloud of the day when the definition of disease would be so broad that his company could "sell to everyone," like chewing gum.
That day is rapidly approaching, if it's not already here. "We're increasingly turning normal people into patients," said Dr. Lisa M. Schwartz of the Dartmouth Medical School. "The ordinary experiences of life become a diagnosis, which makes healthy people feel like they're sick."
In one sense, the ads have been successful. The Kaiser Family Foundation found that every dollar drug companies spend on ads brings more than four dollars in additional sales. But for most others, the result has been soaring medical insurance costs, toxic side effects, and new tensions between doctors and patients, who increasingly badger doctors for the drugs they've seen on TV.
One study found that 30 percent of Americans have made these demands. A Minnesota doctor complained recently that patients now push him for sleep medications "when maybe they just need to go to bed on a more regular basis."
But perhaps the worst part is that prescription drug ads have immersed us all in a pervasive drug culture that seems to have no boundaries. We are being reduced to helpless "consumers" who have no capacity to deal with challenges other than by taking a pill. Last month Tim Pawlenty, the Republican governor of Minnesota, called for a moratorium on prescription drug ads. It's about time.
For most of the past half century, there were tight restrictions on the general advertising of prescription drugs. These require doctors' guidance for a reason; so why should Madison Avenue get involved? But under heavy pressure from the drug and advertising industries, the government backed down in the late 1990s, and that started the tsunami.
Spending on drug ads for the general public more than tripled between 1996 and 2001. It is now some $4 billion a year, which is more than twice what McDonald's spends on ads. In 1994, the typical American had seven prescriptions a year, which is no small number. By 2004, that was up to 12 a year. Homebuilders are touting medicine cabinets that are "triple-wide."
The industry says this is all about "educating" the consumer. But an ad executive was more candid when he said - boasted, really - that the goal is to "drive patients to their doctors." Reuters Business Insight, a publication for investors, explained that the future of the industry depends on its ability to "create new disease markets." "The coming years," it said, "will bear greater witness to the corporate-sponsored creation of disease."
The Kaiser study found that drug ads increase sales for entire categories of drugs, not just the one in question. The ads really are selling the disease more than a cure.
Advertising is just one way the industry has sought to accomplish this goal. It also funds patient advocacy groups such as Children With Attention Deficit Disorder (CHADD), and doctors who push for expanded definitions of disease, among a host of other things. (When the definition of ADD expanded in the 1980s, the number of kids tagged with this problem increased by 50 percent.)
But advertising is the most pervasive and aggressive way of selling sickness. It also is the hardest to justify. Medicine is supposed to be about science, not huckstering; about healing people, not persuading more of them that they are sick. There are far better ways to inform the public about health issues than to spend billions of dollars a year pushing pills.
This is why more than 200 medical school professors recently called for an end to prescription drug ads, and why close to 40 health and seniors groups have joined them. Even the American Medical Association, many members of which have close ties to the pharmaceutical industry, has urged restrictions. Washington should listen to these doctors. As Governor Pawlenty put it, we need to put "the decisionmaking back where it should be - on an informed basis between the patient and the doctor."
Thursday, August 17, 2006
Over 50? - No Flu Vaccine for you!
Ordinarily, people over 65 and those who have chronic illnesses are given priority. As a group, they are the most vulnerable. Protecting the most vulnerable saves the most lives.
But avian flu is not an ordinary flu. Chances are, it will never turn into a global catastrophe, but if it did, some researchers speculate that it could lead to 90 million cases and 1.9 million deaths. There is no way to manufacture enough vaccine in time to protect everyone in the United States. So who should get the potentially lifesaving vaccine?
"This is a tragic choice," says bioethicist Ezekiel J. Emanuel at the National Institutes of Health, who with his colleague Alan Wertheimer suggested an alternative policy in a report in the May 12 edition of Science magazine.
Both the rebel authors and the traditionalists (that is, the government's National Vaccine Advisory Committee and the Advisory Committee on Immunization Practices) would give top priority to those working to manufacture and distribute the vaccine and to front-line health care professionals. And both would give preference to key government leaders. The fight is over what's left for the general public.
The rebels challenge the principle of saving the most lives. What about saving people with the most years yet to live?
Their formula is based on the principle that all people deserve the chance to live out their lives and grow old -- especially teenagers and young adults who have survived childhood and face many decades ahead. Twenty-year-olds, for example, are more "valued than 1-year-olds because the older individuals have more developed interests, hopes and plans but have not had an opportunity to realize them," write the authors.
Presumably, really older individuals -- those over 65 -- have had all the opportunities they need to realize their hopes and plans and interests -- so they are less valued than 20-year-olds.
In the new formula, the winning age cohort in the vaccine rationing sweepstakes would be healthy people 13 to 40. Next in line would be those 7 to 12 and people 41 to 50. After 50, forget it!
If women don't care, who will?
"This feminine ethic of service, marked by attentiveness to the person and concern for their integral (moral and cultural as well as physical) good, is the great contribution women can make to professional life and the workplace in general. To grasp this is to see a solution to the work-life dilemma. Once 'life' -- relationships in the family, with others -- is understood in terms of mutual service, work finds its proper purpose and place."
Who said women are the only ones who care? Shouldn't this "ethic of service" apply to both sexes?
Thursday, August 10, 2006
Yet Another Reason to Support Universal Health Care Coverage: To Protect Our Future
From the Kaiser Network:In Colorado, uninsured children and children enrolled in Medicaid who are hospitalized at Children's Hospital in Denver are twice as likely as those with private insurance to die after hospitalization, according to a study published in the journal Pediatrics, the Denver Rocky Mountain News reports. For the study, two physicians at the hospital looked at hospitalization rates per 100,000 children ages six months to 18 years. The study finds that children enrolled in Medicaid are twice as likely as those with private insurance to be hospitalized for vaccine-preventable illnesses, complications of diabetes and asthma, and ruptured appendices (Brand, Denver Rocky Mountain News, 8/7). Regular preventive care visits by uninsured children and children enrolled in Medicaid would save Colorado $46 million, according to the study. The study also finds that the number of Colorado pediatricians who are willing to see Medicaid beneficiaries fell from 41.4% in 2000 to 23.9% in 2003. Eighty-three percent of Colorado pediatricians in 2003 said Medicaid reimbursements did not cover the cost of office visits (Auge, Denver Post, 8/7). Stephen Berman, head of general pediatrics at Children's Hospital and co-author of the study, said, "The paper, for the first time, provides the data that show there are huge potential savings in caring for these kids." Berman added, "The mortality rates are higher for children on Medicaid. They are higher because they are sicker, and they didn't get their primary care" (Denver Rocky Mountain News, 8/7).
Colorado Children Without Private Insurance Have Higher Mortality Rate
Wednesday, August 09, 2006
America's New Comic Book Superheroine and the Cost of Caring

I love this concept!: America's first true female super-hero since Wonder Woman ~ Carrie Giver. Conceived for the Caregiver Credit Campaign,the feminist superhero will "have politicians and hairdressers, women and girls, hardhats and female executives, right along with caregivers re-thinking personal and social policy, including Social Security. Carrie Giver will be kicking butt in the name of hundreds of millions of people, especially mothers, who give care to the young and old alike each and every day."
More from the designer's website: "This timely comic book reflects the attention now being paid to America's rapidly growing mammogram generation (squeezed on both sides), soon to be in need of care themselves - e.g., aging baby boomers. The trend of first time Hollywood moms, posed against both a right wing view of motherhood-or-nothing and the career-first pressures of still many other women, make this the perfect time for media outlets to talk about the value of caregiving to both children and older people. It affects 100 percent of Americans, and is a worldwide concern as western nations age. We all come into the world in need of care. We all exit the same way. Sooner or later, most of us become caregivers."
An Act of Kindness and Conscience and equitable distribution of resources
Monday, August 07, 2006
Women's Bioethics Project and Center for Women Policy Studies Organizations Launch State Legislative Advisory Board on Women and Bioethics
First Meeting to Convene at the National Conference of State Legislatures in
The advisory board meeting will feature a presentation by Dr. Robin N. Fiore, who is the Adelaide R. Snyder Professor of Ethics at Florida Atlantic University. Dr. Fiore's presentation will cover a full range of bioethics issues ranging from end-of-life issues and the effect of the Terry Schiavo case to stem cell research and its impact on women's reproductive rights and health. Following the presentation, the legislative leaders attending the meeting will assist the Center for Women Policy Studies and the WBP in planning a Bioethics Seminar for Women State Legislative Leaders, which will take place in 2007.
According to Leslie R. Wolfe, Ph.D., president of the Center for Women Policy Studies, "This meeting will provide a forum for state legislators to discuss the challenges they face in understanding the ethical implications of legislative issues pending at the state level." Wolfe, whose organization works with legislators on a range of key policy issues, noted that, "State legislators need support to preserve women's decision-making rights on all these complex issues."
"We are delighted to have such an impressive group of state legislators to advise us. We are focusing on the state legislative level because that's where many of these complicated bioethical issues tend to emerge," said Kathryn M. Hinsch, founder of the WBP. "We firmly believe that women's voices need to be heard, because women are uniquely affected by issues in biotechnology and healthcare. By providing legislative leaders with the support they need to champion these issues, we can help ensure that the entire spectrum of human experience is represented on these critical issues."
The founding members of the state legislative bioethics advisory board are listed here:
-- State Senate Majority Leader Lisa Brown, Wash.
-- State Senator Patrice Arent, Utah
-- State Senator Joan Bray, Mo.
-- State Senator Pam Brown, Neb.
-- State Senator Jennie Forehand, Md.
-- State Senator Karen Fraser, Wash.
-- State Senator Nia Gill, N.J.
-- State Senator Toni Harp, Conn.
-- State Senator Maggie Tinsman, Iowa
-- Former State Senate Majority Leader Lana Oleen, Kan.
-- State Representative Kathy Hawken, N.D.
-- State Representative Linda Lopez, Ariz.
-- State Representative James Roebuck, Pa.
-- State Delegate Jean Cryor, Md.
-- State Secretary of Administration Viola Baskerville, Va.
About the Center for Women Policy Studies
The Center for Women Policy Studies is a Washington, D.C.-based think tank and was founded in 1972. It works with policy makers on such women's human rights issues as reproductive rights and health, international trafficking of women and girls, and the alleviation of women's poverty. (http://www.centerwomenpolicy.org)
About the Women's Bioethics Project
The Women's Bioethics Project (WBP) is an independent, nonpartisan, public-policy think tank based in Seattle. WBP is dedicated to ensuring that women's voices, health concerns and unique life experiences are represented in discussions and decisions about ethical issues in healthcare and biotechnology. (http://www.womensbioethics.org)
Administration to set health care information standards
Monday, August 7, 2006; Page A04
The Bush administration will soon launch an ambitious effort to require that all providers of federally financed health care adopt quality-measurement tools and uniform standards for their information technology, Health and Human Services Secretary Mike Leavitt said Sunday.
The goal of the initiative, Leavitt said, is to reduce health-care cost inflation while increasing the quality of medical services individuals receive.
The executive order would affect doctors and hospitals serving the Medicare population of elderly Americans and people served by any other federally financed service.
It would require those health providers to join with the government to standardize the requirements for information technology systems coming into their facilities; set standards for care of specific health problems; and develop uniform methods of measuring and reporting the outcomes of treatments.
Distribution of Resources Curing Hunger and Malnutrition One Spoonful at a time
Swollen bellies, orange hair, listlessness and dull eyes — these are the traits of child malnutrition in Haiti, the poorest country in the Western Hemisphere and where roughly one of every three children is chronically malnourished.
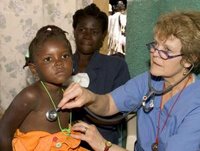
To try to change that statistic, Patricia A. Wolff, M.D., associate clinical professor of pediatrics. at Washington University School of Medicine in St. Louis, founded Meds & Food for Kids (MFK) in 2004, after she saw that medications and small amounts of the local staples rice, beans and corn weren't enough to nourish children back to health.
MFK works to combat childhood malnutrition and related diseases in northern coastal Cap Haitien, Haiti's second-largest city, by giving Ready-to-Use Therapeutic Food (RUTF) to malnourished children between 6 months and 5 years old. The mixture, known to Haitians as "Medika Mamba," or peanut-butter medicine, is a nutrient-rich mixture of peanuts, sugar, oil, vitamins, minerals and powdered milk. It is distributed in plastic containers for families to feed their children at home and can be stored for several months.
Children start to show visible signs of improvement about 1-2 weeks after receiving the peanut-butter mixture, becoming more active and growing new black hair. One course of the six-week treatment, which can be enough to renourish the child, costs under US$100.
For the rest of the article click here.
(Image courtesy of Washington University School of Medicine)
'Fresh Embryos' -- On Sale Now!
The American Society of Reproductive Medicine has been reluctant to invoke standards for fertility clinics, but maybe this latest development will prompt them:
Ethical row over world's first 'made to order' embryos
By Julie Wheldon, Daily Mail 21:46pm 4th August 2006
For around £5,000 couples can buy ready-made embryos matched to their specific requirements - even down to choosing what eye and hair colour they would like their child to have.
In each case the embryos are made from eggs and sperm from two donors who have never even met. The moment of conception occurs in the laboratory and is determined by the genetic combination the clinic thinks will best meet the needs of the paying couples on its books.
Ethical campaigners last night condemned the move as the "absolute commercialisation of human life." They said it was heart-breaking that babies are now being treated as the equivalent of a supermarket "special offer".
Currently in the 
But the new service is totally different as it allows couples to buy fresh embryos that fit their requirements but which have no biological link to either of them.
The human embryo bank is being run by The Abraham Center of Life in
It boasts that its sperm donors all have doctorate degrees and most of its egg donors have college degrees, are under 25 and healthy. So far most of the couples on its waiting lists are happy just to get an embryo and have not set out detailed requirements.
Waiting list for Aryan children
However some have asked for - and been allowed to join list of recipients that will get - embryos made from blond haired and blue eyed donors.
To read the rest of the article, click here.
Tuesday, August 01, 2006
Who Will Care for the US Elderly if the Borders are Closed?
(subscription required) Who Will Care For U.S. Elderly If Border Closes?
By BARRY NEWMAN
July 26, 2006; Page B1
PHILADELPHIA -- Forty years ago, Blanca Maldonado moved to the U.S. from Puerto Rico. She married and had 13 children, 28 grandchildren and 30 great-grandchildren. Now 76 years old and bedridden with bone disease, she is cared for by Xiomara Martinez, an immigrant who arrived in 1996 from the Dominican Republic. Luis Maldonado, a 43-year-old cook, sat at his mother's bedside on a visit to her small apartment here one afternoon. "We'd all love to be with her all the time, but we have to take care of our needs," he said of his family. "But we never stop thinking of Mama." Neither does Ms. Martinez. Her own mother entered the U.S. illegally in 1978 and eventually got a green card. After a long wait, her children joined her. At 47, Ms. Martinez earns $6.65 an hour, paid through a state agency, for taking care of Ms. Maldonado's needs, from baths to rice and beans.
The article goes on to say that in the immigration fight that continues to frustrate Congress and its constituency this summer, workers such as Ms. Martinez are examples that the issue cuts into something more basic: a demographic thundercloud moving over the country as baby boomers approach old age. Immigrants, whether legal or undocumented, make up a disproportionate share of those who care for the elderly -- and the need for such workers is set to explode in the coming years. Where will the extra helpers come from?








